Section 4 Simple descriptive table
Descriptions of key data sources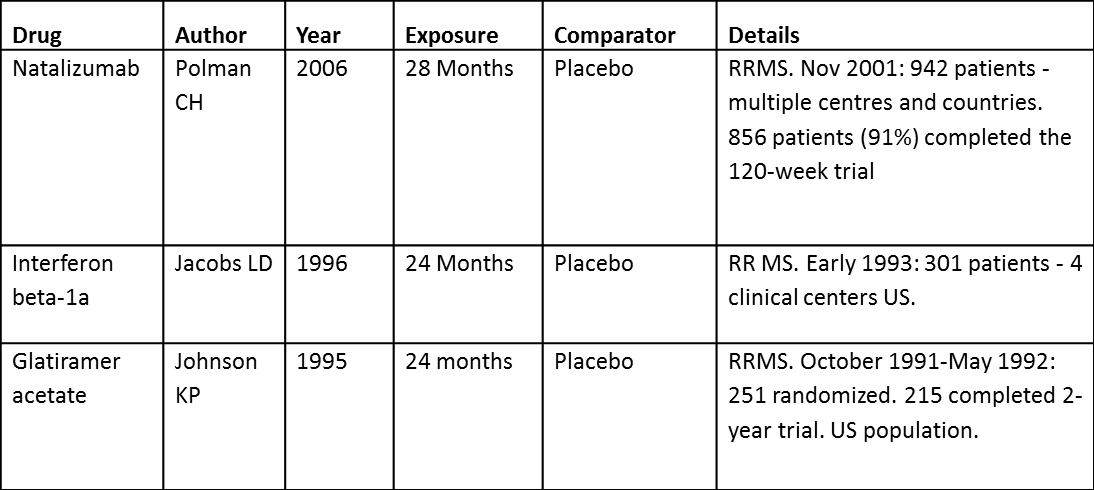
| Short name | Description |
|---|---|
| Name/rubric | Simple descriptive table on included study articles |
| Created in | Microsoft Word (table facility) |
| Message | To lay out descriptive (or quantitative) information in a grid structure. The figure describes the study characteristics; showing larger, more recent, and longer trial was carried out for natalizumab compared to Beta-interferon and Glatiramer acetate. All active drugs used placebo as comparator. |
| Intended audience | Statisticians, regulators, and physicians. Not for patients |
| Knowledge required | Low statistical and low medical knowledge. |
| Unintentional message | The table implicitly assumes that the quality of evidence for all studies is similar and for comparison to be made, and that the populations are also comparable. This may not always be the case. |
| Message not communicated | Characteristics of patients who took part in the trials are not highlighted, implicitly assuming they are comparable |
| Proposed improvement | Display demographics information for patients who took part in the trials and highlight similarities or dissimilarities. |
