Indirect Treatment Comparison (ITC)
We summarisedthe results on each outcome in the model from all studies included in the Rimonabant Wave 2 Case Studyfor the three active comparators and placebo. We fitted random effect Bayesian ITC models using WinBUGS 1.4.3.
Dichotomous outcomes(achieving 10 % weight loss, psychiatric disorders, cardiovascular disorders and gastrointestinal disorders) were approximated by binomial distributions:
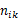 is the number of events observed among
is the number of events observed among  patients, and
patients, and 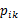 is the risk of events in study i for treatment k. The available data quality did not permit the model to be adjusted for differing study durations. However, the studies included did not vary greatly in their durations.
is the risk of events in study i for treatment k. The available data quality did not permit the model to be adjusted for differing study durations. However, the studies included did not vary greatly in their durations.
We approximated the change in HDL cholesterol by a Normal distribution accounting for study sample sizes and inter-study variations. If
Given the sparse data of risk outcomes, we also used post-marketing studies data in addition to published RCTs. The data we used in the Indirect Treatment Comparison aregiven in Section 12 of the Rimonabant Wave 2 Case Study report.
We derived and presented the posterior distributions for each outcome by treatment in tables below.Using placebo as the common comparator, the treatment effects of the three comparators are listed in Table 1 below.
Table 1 Odds ratios [95%CI], unless otherwise specified, from the indirect treatment comparison model
| Criteria | Placebo | Orlistat | Sibutramine | Rimonabant |
|---|---|---|---|---|
| 10% Weight loss | 1 | 2.38 [1.93,2.95] | 6.91 [3.66,13.02] | 5.18 [3.61,7.44] |
| HDL Cholesterol(Mean difference, mg/dL) | 0 | 0.90 [0.64,1.26] | 1.84 [1.00,3.40] | 1.10 [0.53,2.30] |
| Cardiovascular disorders | 1 | 6.09 [0.01,4,843.53] | 2.34 [0.02,232.42] | 1.50 [0.04,57.93] |
| Psychiatric disorders | 1 | 0.30 [0.11,0.81] | 1.64 [0.71,3.79] | 1.69 [0.96,2.96] |
| Gastrointestinal disorders | 1 | 4.11 [2.30,7.32] | 1.75 [0.94,3.27] | 1.41 [1.02,1.94] |
We converted the odds ratios and mean difference in Table 1 above into rates and absolute measurement to be used in the multi-criteria decision models. The results are shown in Table 2 below.
Table 2 Median of rates of event [range], unless otherwise specified, from the indirect treatment comparison model
| Criteria | Placebo | Orlistat | Sibutramine | Rimonabant |
|---|---|---|---|---|
| 10% Weight loss | 0.11 [0.10,0.14] | 0.24 [0.15,0.34] | 0.47 [0.16,0.80] | 0.40 [0.22,0.62] |
| HDL Cholesterol(Mean difference, mg/dL) | 1.02 [0.95,1.10] | 1.92 [1.12,2.75] | 2.63 [1.19,4.11] | 2.12 [0.26,3.93] |
| Cardiovascular disorders | 0.02 [0.01,0.02] | 0.11 [0.00,1.00] | 0.04 [0.00,1.00] | 0.03 [0.00,0.99] |
| Psychiatric disorders | 0.01 [0.01,0.01] | 0.00 [0.00,0.03] | 0.02 [0.00,0.11] | 0.02 [0.00,0.06] |
| Gastrointestinal disorders | 0.05 [0.05,0.06] | 0.19 [0.06,0.50] | 0.09 [0.02,0.35] | 0.07 [0.04,0.15] |
