An example of an "effects table"
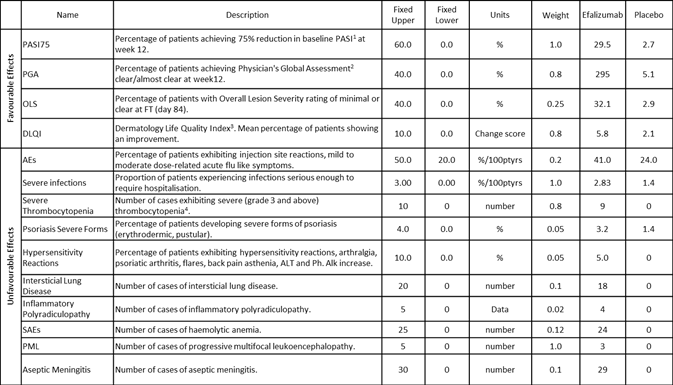
The 'effects table' above shows a list of the criteria for the benefit-risk assessment model in the Efalizumab Case Study. The structure of an effects table is based on the PrOACT-URLframework and MCDA. It shows an overview of numerical values of thecriteria for different alternatives. When setting up a benefit-risk assessment problem, specialised tables like this were found to be useful in PROTECT case studies. Such structured tables provide increased consistency and clarity to the decision problem. Whilst the type of information to include on an effects table is suggested, there is some flexibility to add or omit information relevant or not relevant to the decision problem.
