What is benefit-risk assessment?
What are benefits?
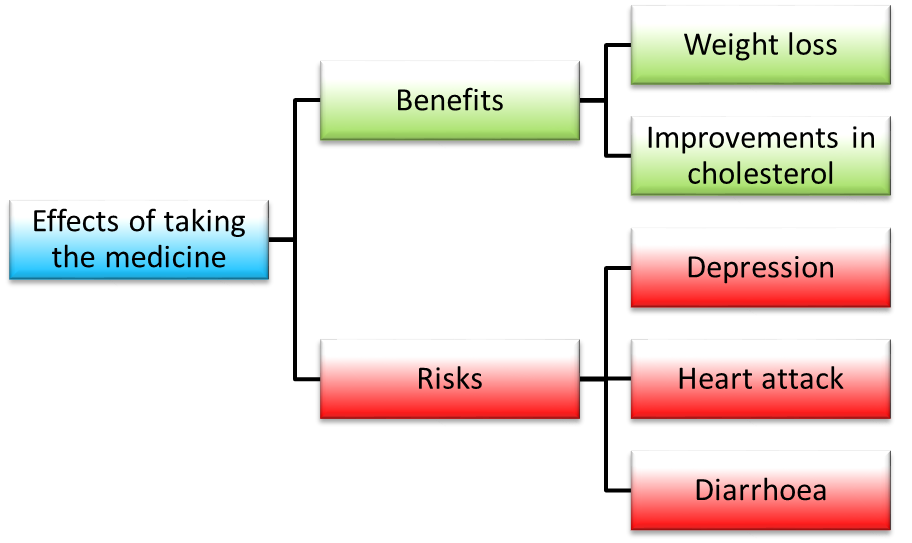
In PROTECT benefits can be referred to as “favourable effects”. These are positive outcomes which may happen after taking a medicine. For example, one of our case studies which looked at treating obesity found the positive outcomes of treatment to be:
- (1) Losing ten percent bodyweight or more
- (2) An improvement in cholesterol levels
What are risks?
In PROTECT risks can be referred to as “unfavourable effects”. These are unintended negative outcomes which may happen when a patient takes a medicine. Often people refer to them as side effects or adverse events. Using the obesity treatment example, we found the negative outcomes of treatment to be:
- (1) Cardiovascular adverse events (e.g. stroke and heart attacks)
- (2) Psychiatric adverse events (e.g. depression)
- (3) Gastrointestinal adverse events (e.g. diarrhoea)
How is the benefit-risk balance of a medicine currently assessed in the European Union?
In the European Union, the European Medicines Agency (EMA) is the overall regulatory authority responsible for evaluating medicines for human use that are authorised through the centralised procedure. The EMA coordinates the scientific assessment of medicines carried out by its scientific committees which are made up of members nominated by national regulatory agencies across each European Member State (such as the Medicines and Healthcare Products Regulatory Agency or the MHRA in the UK) as well as independent scientific experts and representatives of patient, consumer, and healthcare professional organisations. Medicines that do not fall within the scope of the centralised authorisation procedure are marketed in accordance with national, decentralised, or mutual recognition procedures. These are overseen by individual member states or the Coordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh).
The decision to approve a medicine to treat specific diseases in patient populations depends on a range of factors. One of the key considerations when granting a license is how the benefits of a given treatment balance against the risks. If the balance of benefits outweighs the potential risks, then a medicine may be granted a license so that it can be made available to patients through a clinician via a prescription, or be purchased over-the-counter without a prescription. If the balance of benefits is outweighed by the risks, then this will lead to a medicine not being approved for use in patients.
The balance of benefits and risks for a medicine can change over time as new knowledge and data accumulated regarding the effects of a medicine when used in the wider population. Regulatory agencies continuously monitor medicines for any safety concerns so as to protect patients and public health.
What are systematic methods of benefit-risk assessment?
When regulators have to make a decision about the balance of benefits and risks, these decisions are often complex. The rationale behind why the final decision was positive or negative may be challenging to communicate. The use of systematic methods can help by providing step by step instructions for making decisions that clearly show how benefits and risks have been considered. Systematic methods can also be used to show how patients and the public value different benefits and risks of medicines.
