Q-TWiST (Quality adjusted Time Without Toxicity and Symptoms)
1. Description
Q-TWiST (Quality-adjusted Time Without Symptoms and Toxicity) is in principle a QALY metric, with explicit definitions of the discrete health states in cancer therapy: toxicity, time without symptoms and toxicity, and relapse.[1][2] The resultant time-utilities in the three health states are recombined using utility-weighted sum .
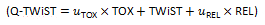
2. Evaluation
2.1 Principle
- Q-TWiST is an individual level metric.
- Variability of Q-TWiST from different treatment or population is handled using standard statistical analysis.
- The utilities that make up Q-TWiST are typically judged by the clinicians based on the disease and treatment.
- Q-TWiST can deal with any number of treatment options but is confined to survival endpoints only.
2.2 Features
- Benefits and risks are naturally integrated and time dimension is also incorporated.
- Sensitivity analysis can be performed on the impact of different utilities on the Q-TWiST metric.
- Incorporating multiple sources of evidence using Q-TWiST certainly requires all sources of data to have the Q-TWiST derived in the same way.
2.3 Visualisation
- Q-TWiST is visualised as stratified survival curves.
2.4 Assessability and accessibility
- The concept of Q-TWiST is easy to understand.
- Value preference is captured in the utility of Q-TWiST derivation.
- Although Q-TWiST was developed within the oncology domain.
- Its application can be generalised to other diseases or medical conditions with similar characteristics, e.g. in measuring quality of life in patients with HIV infections, [3], multiple sclerosis and epilepsy.[4]
3. References
[1] Gelber RD, Cole BF, Gelber S, Aron G. Comparing Treatments Using Quality-Adjusted Survival: The Q-Twist Method. The American Statistician 1995 May 1;49(2):161-9.[2] Goldhirsch A, Gelber RD, Simes RJ, Glasziou P, Coates AS. Costs and benefits of adjuvant therapy in breast cancer: a quality-adjusted survival analysis. J Clin Oncol 1989 Jan;7(1):36-44.
[3] Gelber RD, Lenderking WR, Cotton DJ, Cole BF, Fischl MA, Goldhirsch A, et al. Quality-of-Life Evaluation in a Clinical Trial of Zidovudine Therapy in Patients with Mildly Symptomatic HIV Infection. Annals of Internal Medicine 1992 Jun 15;116(12 Part 1):961-6.
[4] Schwartz CE, Cole BF, Gelber RD. Measuring Patient-Centered Outcomes in Neurologic Disease: Extending the Q-TWiST Method. Arch Neurol 1995 Aug 1;52(8):754-62.


