FDA BRF (The US FDA Benefit-Risk Framework)
1. Description
The FDA BRF addresses the issues relevant to regulatory decision making. It aims to aid communication within and outside the FDA by approaching benefit-risk decision making through a set of guidelines that are described as simple and user-friendly; can address critical issues; are capturing expert views faithfully; can represent issues transparently; are compatible with quantitative analysis of clinical benefit and risk; can facilitate communications; and are broadly applicable. FDA BRF would "tell the story" of a decision problem through the framework.
2. Evaluation
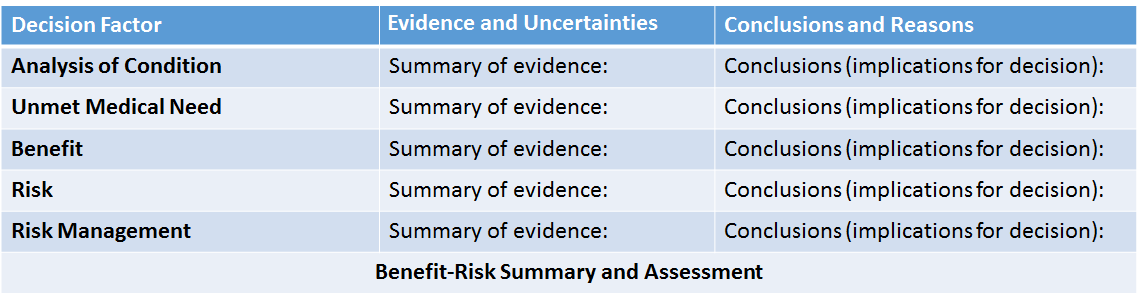
FDA BRF focusses on structured qualitative approach that provides a high-level snapshot and the concise bottom-line descriptions of the relevant issues to the regulatory decision in words. FDA BRF asks five relevant questions:- "what is the problem?";
- "what other potential interventions exist?";
- "what is the benefit of the proposed intervention?";
- "what am I worried about?";
- "what can I do to mitigate/monitor those concerns?"
3. References
[1] Jenkins J. A United States Regulator's Perspective on Risk-Benefit Considerations. http://www.fda.gov/downloads/AboutFDA/CentersOffices/CDER/UCM210155.pdf. Rockville, MD: Shady Grove Conference Center; 2010.[2] Frey P. Benefit-risk considerations in CDER: Development of a Qualitative Framework. http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM317788.pdf. 28-6-2012. Philadelphia, PA, US Food and Drug Administration.
