Welcome to the PROTECT Benefit-Risk Website
PROTECT, the Pharmacoepidemiological Research on Outcomes of Therapeutics by a European Consortium, contains a number of work programmes whose goal is to strengthen the monitoring of the benefit-risk balance of medicines in Europe and to enhance early detection and assessment of adverse drug reactions from different data sources.
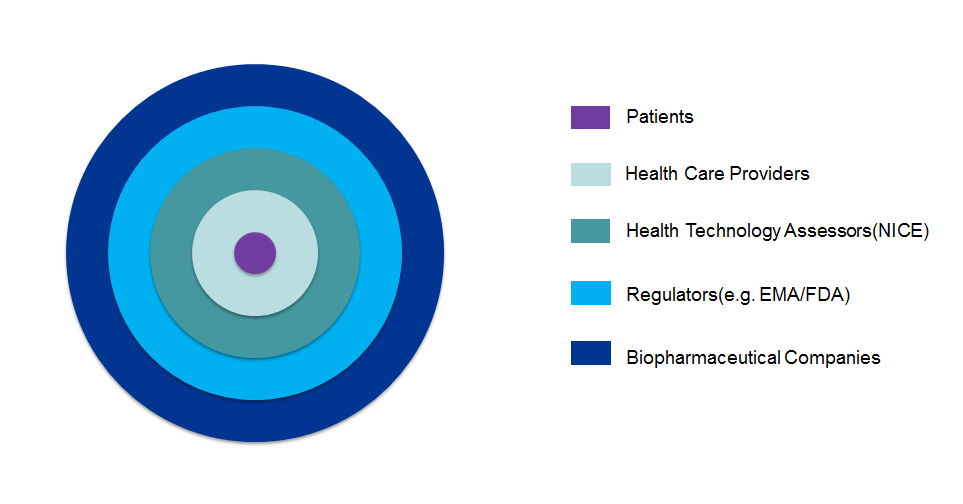
The evaluation of the balance between benefits and risks of drugs is fundamental to numerous stakeholders including patients, healthcare providers, health technology assessors, regulators and biopharmaceutical companies.
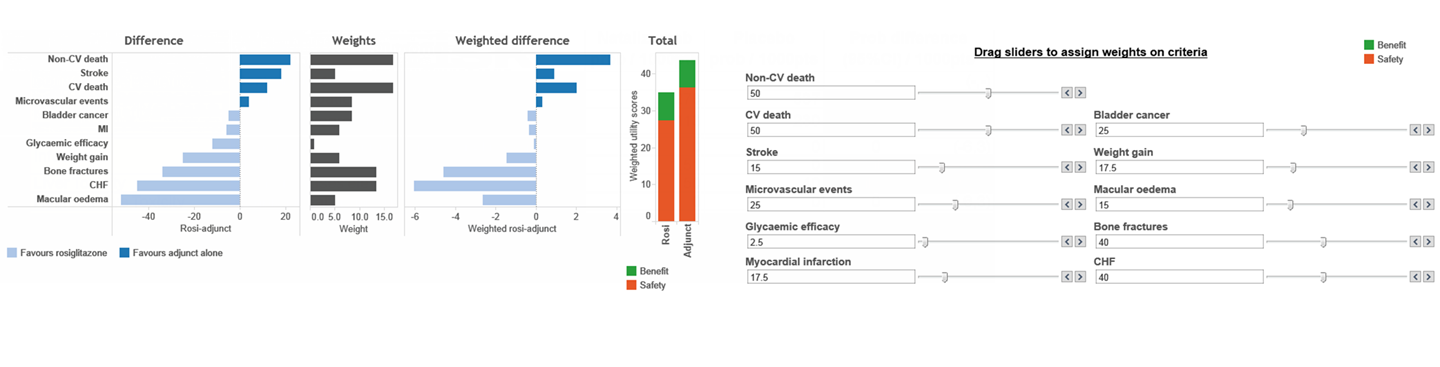
Decision-making with regards to benefit-risk assessment is often complex. It is important to ensure transparent, robust and comprehensive methodologies are used, and also that patient and public preferences on benefits and risks feed into the decision-making process.
Decision Makers - Who are they?
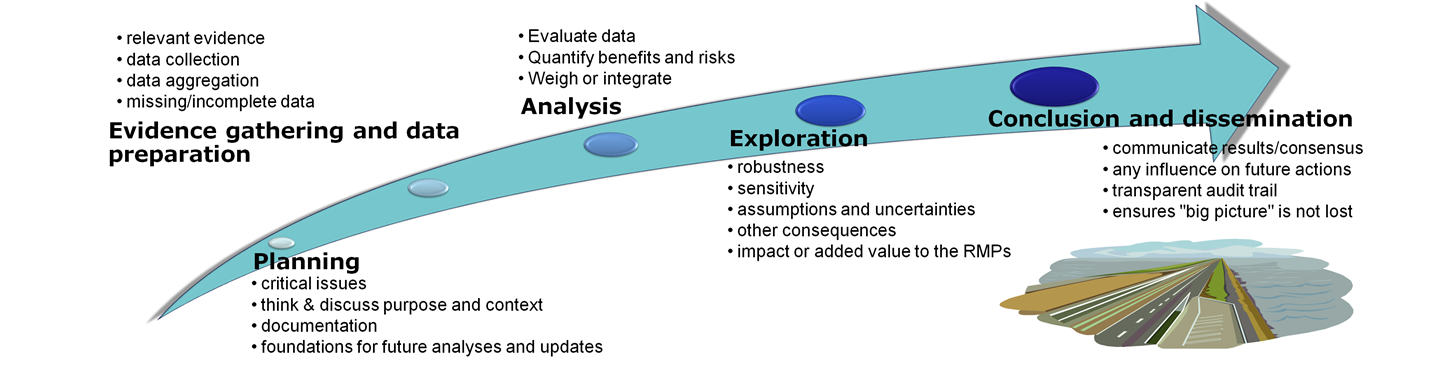
For clarity, these practical recommendations are organised around the five stages of a generic benefit-risk assessment roadmap.

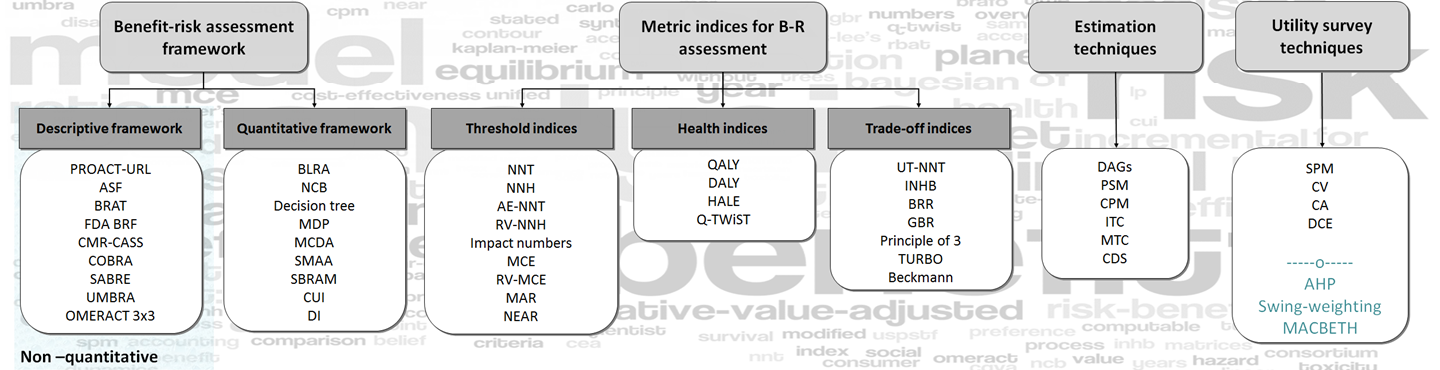
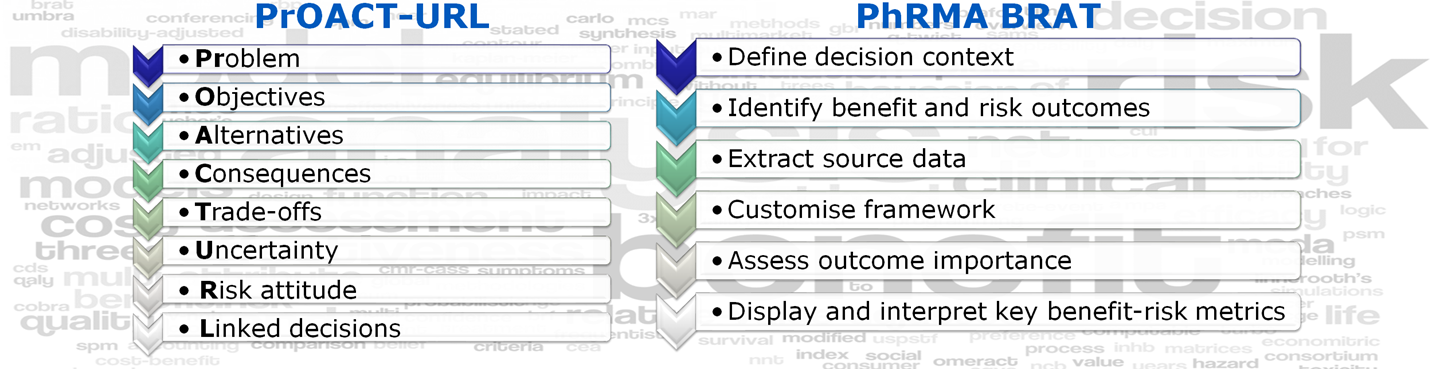
The generic benefit-risk assessment roadmap is underpinned by earlier reports from the Benefit-Risk group. To read the summary findings from the reports, click on the respective boxes below.